When an experiment is performed the column will be first filled with water. 50ml of trichloroethylene is being mixed with 50ml water in conical flask.
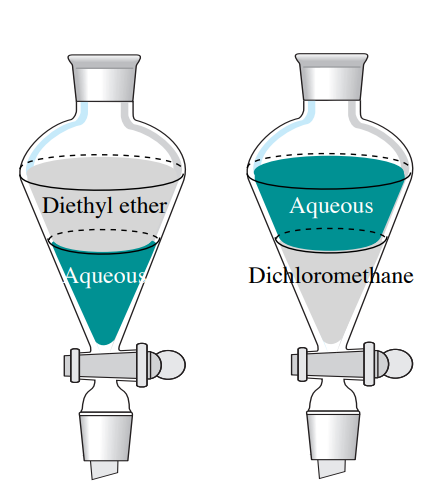
4 4 Which Layer Is Which Chemistry Libretexts
Two phases should be observed.
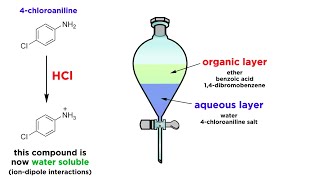
. To make the separation of two liquids in liquidliquid extraction chemists use a separatory funnel. Another common term for Liquid-Liquid extraction is as solvent extraction process. The equilibrium constant for this process is called the partition coefficient or distribution coefficient and is given by.
A stopper is placed and the mixture is shaken for 5 minutes. Use a separatory funnel to separate the two layers. Continuous extraction and distillation experiments are performed to test the extraction power and recovery of 2-MTHF from the extract.
Do not shake vigorously or an emulsion might form which is difficult to separate. Place the cap on the separatory funnel. PROCEDURE FOR EXTRACTION The procedure for carrying out an extraction is quite simple.
This is the continuous phase. Each of the bottom and upper samples is titrated against 01M NaOH using phenolphthalein as the indicator. Then 2ml of propionic acid is added to the mixture.
O Liquid-Liquid Extraction Theory. 7 This process is done by injecting small amounts of an appropriate extraction solvent C 2 Cl 4 and a. It also has applications in the isolation of natural products as in the extraction of caffeine from tea leaves.
2 Generally you want to use the minimum amount of the first solvent to completely dissolve your original mixture containing the two compounds. Liquid-Liquid Extraction The extraction technique can be used to purify compounds or to separate mixtures of compounds such as when isolating a product from a reaction mixture known as an extractive work-up. Students also viewed Experiment 2 Introduction to Wireshark and Cisco Packet Tracer.
Home Create Flashcards Science Experiment Can You Answer Following Questions On Liquid. Liquid Liquid Extraction Experiment Lab Report University Illinois Institute of Technology Course Chemical And Biological Engineering Laboratory Ii CHE 418 Uploaded by Kailee Buttice Academic year 20202021 Helpful. Learn everything related to the Liquid Liquid Extraction Theory with the help of our flashcards quizzes with ease.
LLE is widely used in sample preparation for cleanup and enrichment which results in signal enhancement. Liquid-Liquid extraction is a more complex process of separating a liquid mixture over the Liquid-Solid process. The VLE and LLE data are.
Steps in a liquid-liquid extraction performed with a separatory funnel. The process involves taking liquids mixing them and being able to separate them when the liquid settles. Dispersive liquidliquid microextraction DLLME edit A process used to extract small amounts of organic compounds from water samples.
Ad Find this and more substances to complete your research. To perform an extraction simply combine the original solution and the extracting solvent. Make sure the vial is closed and shake gently by turning the vial upside down and vice versa a few times.
Once the target analytes are in the organic phase they can be re-extracted into a fresh aqueous phase whose pH has been manipulated to ensure the analytes are in the charged form and therefore most highly. Add the extraction solvents to the separatory funnel be certain the Teflon stopcock is closed first. The mixture is then separated using the separation funnel.
Be careful adding the liquid to the separatory funnel. Liquid - liquid extraction also known as partitioning is a separation process consisting of the transfer of a solute from one solvent to another the two solvents being immiscible or partially miscible with each other. Answer these quiz based flashcards based on the Liquid Liquid Extraction Theory Questions and check your knowledge.
Liquid-Liquid Extraction Theory This web-page provides important information on liquid-liquid extraction theory and related issues. 1 The total volume of solvent put into the separatory funnel should never exceed 23 of the total funnel volume. LIQUID LIQUID EXTRACTION CHE523 EXPERIMENTAL PROCEDURE Experiment Part A 1.
Expertise on every level to craft science technology solutions in life science. These are shown in Figure 6-4. Liquidliquid extraction LLE is based on the principle that a solute or an analyte can distribute itself in a certain ratio between two immiscible solvents usually water aqueous phase and organic solvent organic phase.
For the separation of dilute FA solutions liquid-liquid extraction is preferred over conventional distillation because distilling large amounts of water is very energy-intensive. The selectivity of the extraction experiment involving pH manipulation can be further improved using a technique known as Back Extraction. As you shake pressure might build up inside the vial.
The mixture is then separated using the separation funnel. Acid-base extractions are based on solvents of different pH levels to separate acids bases and neutral compounds from each other and when adding an acid or base the compounds become more protonated which also makes. A stopper is placed and the mixture is shaken for 5 minutes.
Frequently one of the solvents is water or an aqueous mixture and the other is a nonpolar organic liquid. An acid-base extraction is a type of liquid-liquid extraction involving an organic solvent being ethanol in this experiment. Liquid-Liquid Extraction LLE Extractionis a process where one or more solutesare removedfromoneliquid phase technically called a diluent by transferring thatthose the solutes toanotherliquid phase or asolvent Since this is the operation between the two liquid phases no vaporisationis needed.
Remember to close the stopcock. Then 2ml of propionic acid is added to the mixture. Liquid-Liquid Extraction Your prelab for this experiment will need to be more detailed than just the steps in the flowchart above since each extraction involves multiple steps.
Then the butyl acetateacetone feed stream will be introduced as the. Holding the cap and funnel securely invert the separatory funnel. The theory of liquid-liquid extraction is based on the equilibrium between the concentrations of dissolved component in the two immiscible liquids when they are in contact.
Often one part is water while the other can.

Structural Biochemistry Organic Chemistry Methods Of Separation And Isolation Wikibooks Open Books For An Open World

Liquid Liquid Extraction Vs Solid Phase Extraction

Liquid Liquid Extraction An Overview Sciencedirect Topics
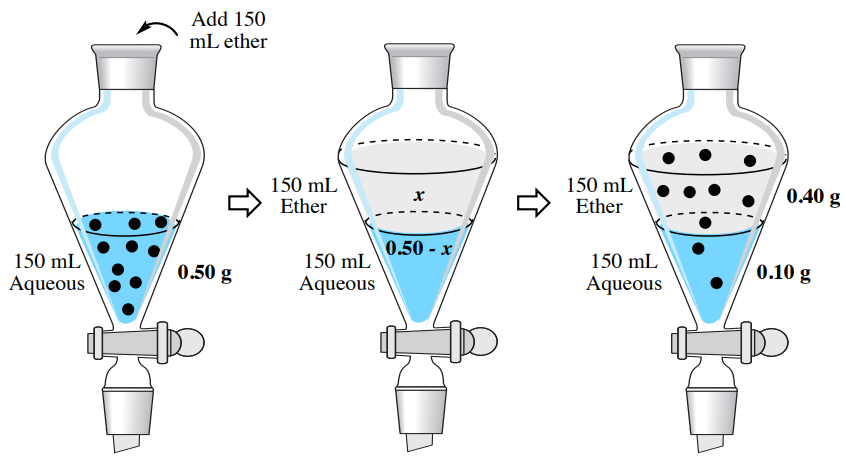
4 5 Extraction Theory Chemistry Libretexts

Liquid Liquid Extraction Youtube
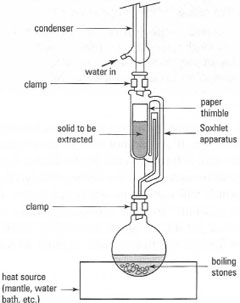
Solid Liquid Extraction Solvent Extraction Laboratory Techniques
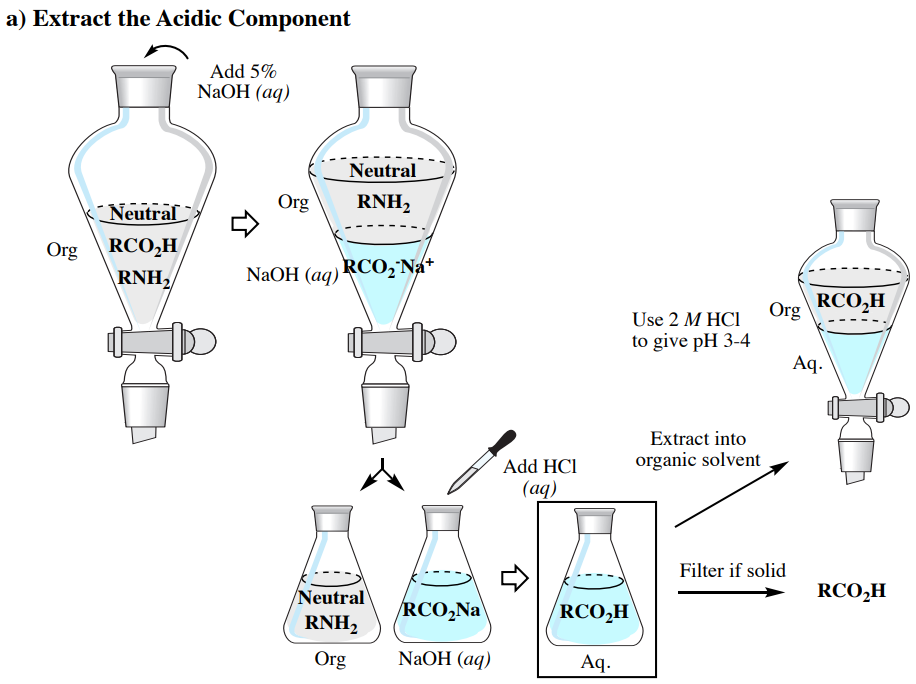
4 8 Acid Base Extraction Chemistry Libretexts

Liquid Liquid Extraction An Overview Sciencedirect Topics
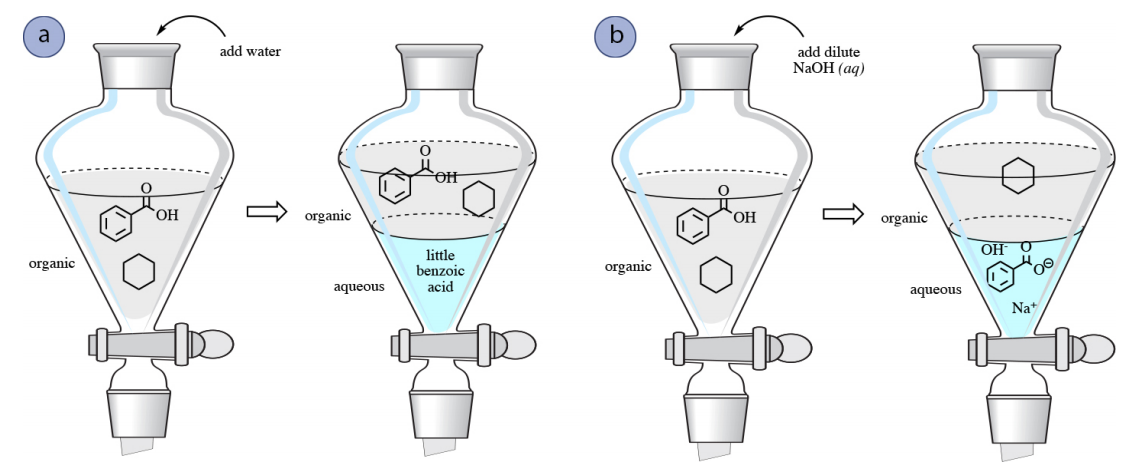
4 8 Acid Base Extraction Chemistry Libretexts
The Complete Guide To Hydrocarbon Extraction New In 2022

Liquid Liquid Vs Supported Liquid Vs Solid Phase Extraction

Solvent Extraction An Overview Sciencedirect Topics
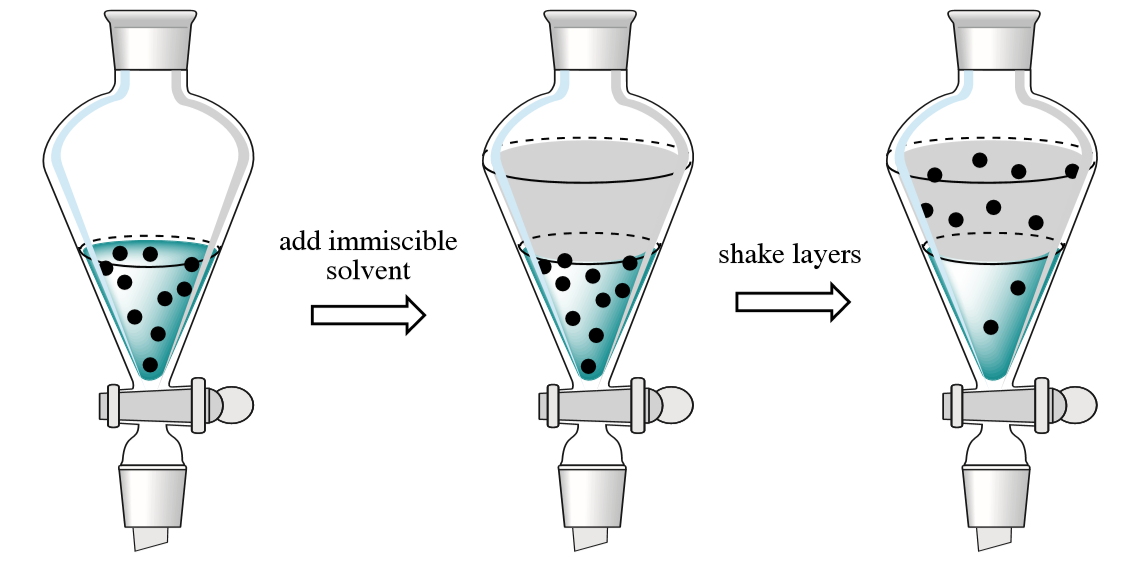
4 2 Overview Of Extraction Chemistry Libretexts

Liquid Liquid Extraction Vs Solid Phase Extraction
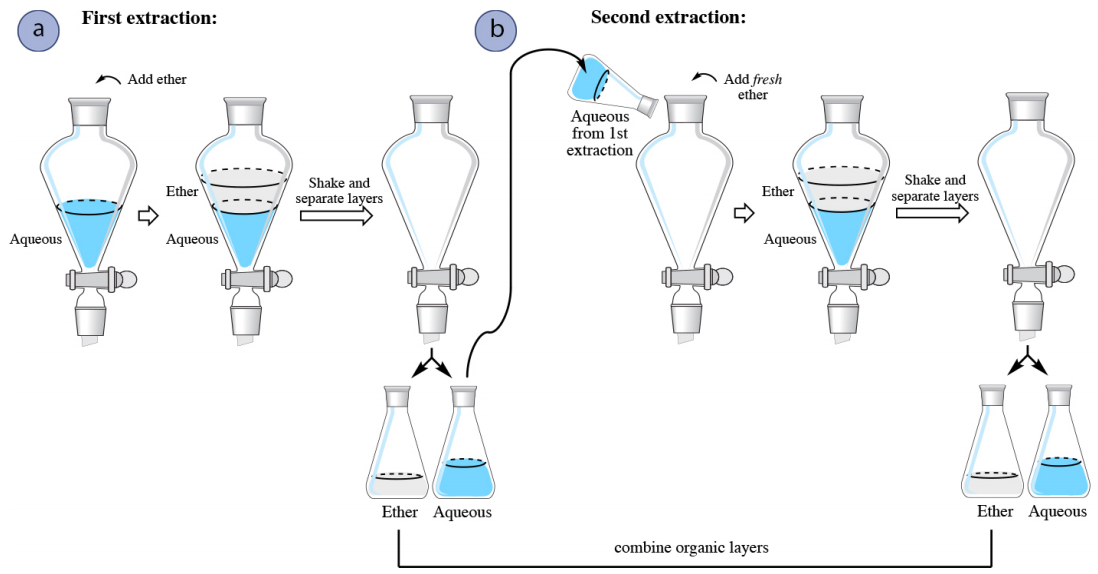
4 5 Extraction Theory Chemistry Libretexts

Separating Components Of A Mixture By Extraction Youtube

Chem117 04 Liquid Liquid Extraction Fundamentals Youtube
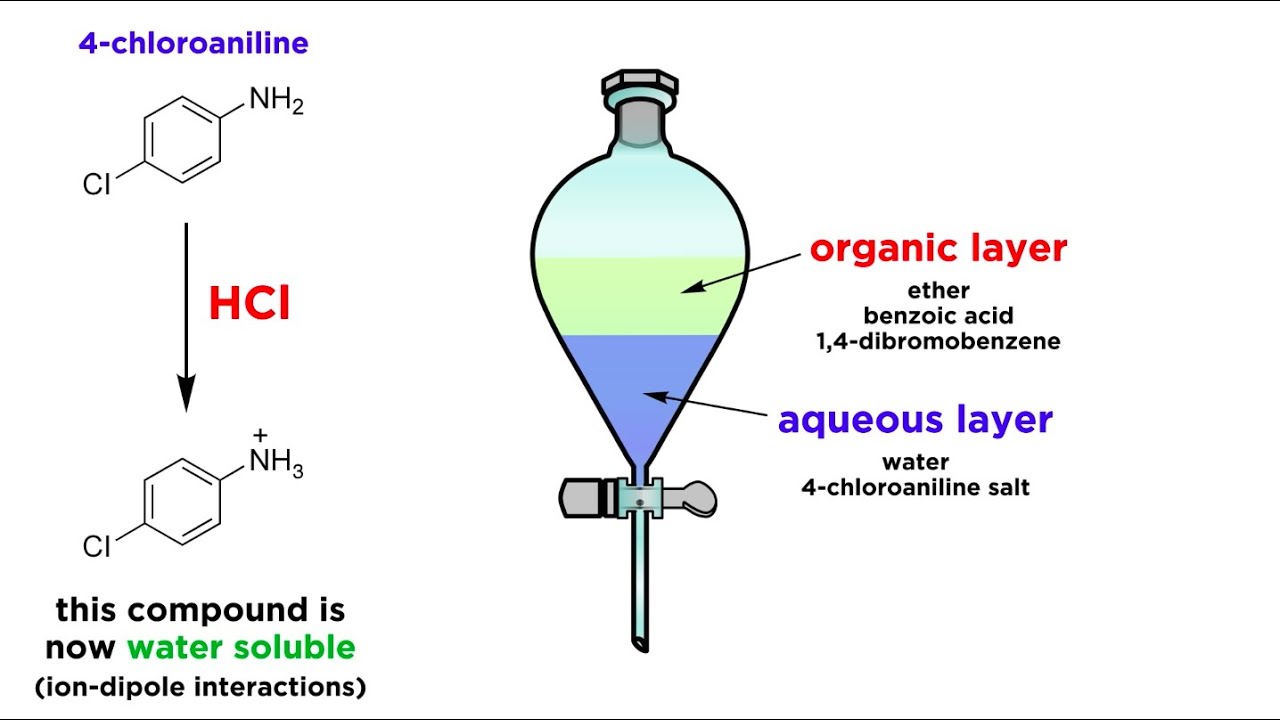
Separating Components Of A Mixture By Extraction Youtube
